Venus Viva Receives FDA Approval for Skin Resurfacing
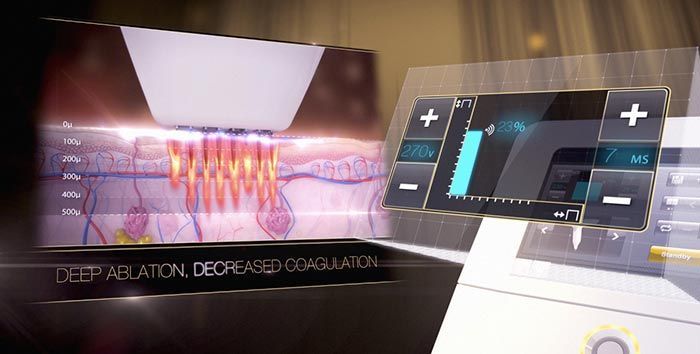
Already a leader in the international medical aesthetics field among the cutting edge plastic surgeons, dermatologists, and aesthetic clinics throughout Europe, Asia, and Canada, the Venus Viva™ will now make its American debut. In a recent press release , Chairman and CEO Domenic Serafino expressed his views for the Venus Viva™ launch:
“Based on our global clinical experience and market acceptance, Venus Viva™ has already established itself as ‘best in class’ technology,” says Domenic Serafino,
This exciting news comes on the heels of an already impressive 2015 for us at Venus Concept, with treatments now being performed in over 50 countries worldwide, and over 3 million treatments each year.
The Venus Viva™ is safe for all skin types, producing incredible clinical outcomes in diverse markets, a major advantage as consumer demand for minimally invasive procedures increases. Patients and physicians alike have been thrilled to share impressive testimonials, reviews, and before and after transformations . Both the patient, and operator experience is paramount, with patients able to seamlessly integrate treatments into their daily lives, and operators having precise customization and control to achieve consistent clinical outcomes.
To learn more about the Venus Viva™ and be one of the first to demo this revolutionary device.








